Off-Topic :
Fairfax Underground

Welcome to Fairfax Underground, a project site designed to improve communication among residents of Fairfax County, VA. Feel free to post anything Northern Virginia residents would find interesting.
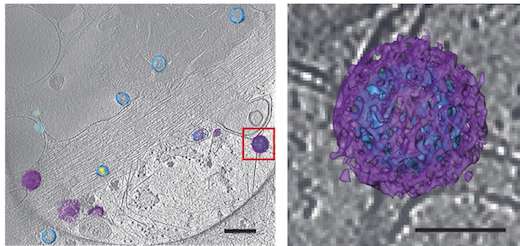
the reader should realize that "ALLOT OF PUBLISHED SCIENCE REVOLVES AROUND DEVELOPING THE BETTER MICROSCOPE" (and who will steal the data and produce it and sell it to governments)
that is no joke. any college lab worth mentioning has not one but many "better microscope" projects ongoing

there are systems designed to look at EM photos for rapid identification - but they are slow, have a thin database of "known likenesses" to identify, and it takes "some intelligence" to identify certain things from certain other things in photos
they are "still in the works" - meaning a lab person is usually better off not having EM rapid identification software

Welcome to Fairfax Underground, a project site designed to improve communication among residents of Fairfax County, VA. Feel free to post anything Northern Virginia residents would find interesting.
more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
breitbart
()
Date: March 22, 2020 05:21PM
https://www.fda.gov/media/136314/download
BELIEVE ME, IF IT'S OBAMA CARE APPROVED, IF IT COSTS MONEY AND GOVERNMENT PAID TO CREATE A WEBSITE: IT'S A FRAUD OF SOME KIND
Cepheid
904 Caribbean Drive Sunnyvale, CA 94089 USA Phone: +1 408 541 4191 Fax: +1 408 541 4192
AH, so California is ALREADY GETTING GOVERNMENT FUNDS for private businesses. and lying about results no doubt. i guess they are also getting money for sales even though no one has asked for the product: i'm quite sure of it

LET ME TELL YOU ABOUT MOLECULAR TESTS - THEY DON'T COME IN A BOTTLE
YOU BUY A SUB-MILLION $ OR MULTI-MILLION $ LAB EQUIPMENT. You buy a data set (which comes with updates). the results (which use one of many standard solutions) have a data pattern - these patterns are compared against a known set (a prisoner up for murder dna, rna etc).
however - a dna rna tester REQUIRES your sample include the dna rna you are looking for (ie, not dna rna of SOMEONE ELSE)
it has nothing to do with buying a small bottle per say
IT IS A NJ BASED BUSINESS RUNNING IN CALIFORNIA? ALREADY SOME QUESTIONABLE LEADS.
Cost[edit]
Some concerns have been raised about the Xpert MTB/RIF, including minor operational issues and cost. The concessional price for a GeneXpert system is currently USD 32,000 for a four module instrument. As of 6 August 2012, the cost of a test cartridge in countries eligible for concessional pricing is USD 9.98.[11] As of 30 June 2013, 1,402 GeneXpert systems (comprising 7553 modules) and >3 million Xpert MTB/RIF cartridges had been procured in 88 of the 145 countries under concessional pricing
------------------------
most sunnyvale california companies have become MADE IN CHINA
they are called "fabless" and it means: they are talking heads, they are importers, they promote the product as american as long as they can get away with doing so
-------------------------
Product: dna
Results per Page
New Searchexport reports to excelExport To Excel | HelpHelp
Product DescriptionSort by Product Name [A-Z]
Sort by Product Name [Z-A] Recall
ClassSort by Recall Class [0-9]
Sort by Recall Class [9-0] FDA Recall
Posting DateSort by Date Classified [0-9]
Sort by Date Classified [9-0] Recalling FirmSort by Recalling Firm [A-Z]
Sort by Recalling Firm [Z-A]
DIASTAT(R) ANA (Anti-Nuclear Antibody) EURO DIAGNOSTICA, The DIASTAT(R) Anti-Nuclear Antibody (ANA) ... 3 10/11/2018 Euro Diagnostica AB
BD MAX DNA MMK Lab Use, Catalog No. 442828 3 04/28/2018 Becton Dickinson & Co.
BD MAX DNA MMK (SPC) For Laboratory Use, Catalog No. 442829 3 04/28/2018 Becton Dickinson & Co.
EnLite Neonatal TREC Kit;An In Vitro Diagnostic Device Intended For The Semi-Quantitative Determinat... 3 12/22/2016 PerkinElmer Health Sciences, Inc.
Thermo Scientific P21 WF1 Ab-3 (DCS-60.2) 1 Ml (0.4mg/Ml): Product Code: MS-230-P And MS-230P0; ... 3 11/20/2015 Lab Vision Corporation
Xpert CT/NG Urine Specimen Collection Kit Part Number GXCT/NGURINE-50; Microbiology: Xpert CT/NG... 3 08/20/2015 Cepheid
Xpert CT /NG Vaginal/Endocervical/ Specimen Collection Kit: Part Number CT/NGSWAB-50; Microbiolo... 3 08/20/2015 Cepheid
Cobas 4800 KRAS AMP/DET 24T CE-IVD Mutation Test; CE-IVD 5852170190. Intended For The Identificat... 3 06/26/2015 Roche Molecular Systems, Inc.
Cobas® EGFR Mutation Test Epidermal Growth Factor Receptor (EGFR) Gene DNA Assay 3 03/25/2015 Roche Molecular Systems, Inc.
The CEP 8 SpectrumGreen (SG) ASR Probe Kit, 20ul (List 06J37-018) Is An Analyte Specific Reagent (AS... 3 04/23/2014 Abbott Molecular
Cobas 4800 BRAF V600 Mutation Test For In Vitro Diagnostic Use Roche Molecular Systems, Inc., Pr... 3 11/02/2012 Roche Molecular Systems, Inc.
Cobas KRAS Mutation Test For In Vitro Diagnostic Use Product Usage: Usage: The Cobas KRAS Mutat... 3 08/08/2012 Roche Molecular Systems, Inc.
Abbott RealTime CT/NG Assay, List 8L07, In Vitro Diagnostic; Abbott Molecular Inc., Des Plaines, IL ... 3 02/23/2011 Abbott Molecular
Abbott RealTime HBV Assay, List 2N40, In Vitro Diagnostic; Abbott Molecular Inc., Des Plaines, IL 60... 3 02/23/2011 Abbott Molecular
Vysis LSI ATM/P53: D13S319 /CEP 12/13q34 DNA Probe Set; Fluorescence In Situ Hybridization (FISH) An... 3 03/13/2007 Abbott Molecular
Vysis LSI P16 (9p21)/CEP 9 (9p11-Q11) Dual Color Probe Set; A Locus Specific Identifier DNA Probe Co... 3 01/24/2006 Abbott Molecular
Anti-DsDNA [125I] Radiobinding Assay Kit. For In-Vitro Diagnostic Use Catalog Number: NEA 103 3 08/20/2003 Perkinelmer Life Sciences, Inc.
Kit BD Max ExK DNA 1 EU LUO; Catalog # 442818 2 08/22/2019 Becton Dickinson & Co.
Kit BD Max ExK DNA 1 USA; Catalog # 442817 2 08/22/2019 Becton Dickinson & Co.
Kit BD Max ExK DNA 2 USA; Catalog # 442819 2 08/22/2019 Becton Dickinson & Co.
Kit BD Max ExK DNA 2 EU LUO; Catalog # 442820 2 08/22/2019 Becton Dickinson & Co.
RNeasy DSP FFPE Kit (48), REF 73604 - Product Usage: The RNeasy DSP FFPE Kit Is A System Intended Fo... 2 06/28/2019 Qiagen Sciences, Inc.
Oncomine Dx Target Test, (Model No. A32451), Oncomine Dx Target RNA/DNA Panel (Model No. A32441). ... 2 04/20/2018 Life Technologies Corporation
Oncomine Dx Target Test User Guides And Assay Definition File, Model: A32461; UDI: (01)1019030200607... 2 03/30/2018 Life Technologies Corporation
DIASTAT Anti-Nuclear Antibody (ANA) / DIASTAT ANA ELISA. Catalog Number: FANA200. Product Usage: ... 2 03/08/2018 Euro Diagnostica AB
Dial-A-Dose Insulin Delivery Device (Pen-Injector) Cartridge Holders Product Usage: The NovoPen ... 2 12/07/2017 Novo Nordisk Inc
EMAG System, Ref 418591 It Is An In Vitro Diagnostic Medical Device Intended For The Automated Is... 2 09/12/2017 BioMerieux, Inc.
Welch Allyn ProBP 2400 Digital Blood Pressure Device #2400, REF 901096, Rx ONLY, Made In China --- D... 2 04/11/2017 Welch Allyn Inc
Cobas 6800/8800 System Hepatitis Viral B DNA Detection Product Usage: The Cobas P512 Pre-Analyt... 2 10/25/2016 Roche Diagnostics Operations, Inc.
Cobas EGFR Mutation Test V2 Materials Number CE-IVD: EGFR V2: 07248563190 CfDNA: 07247737190 ... 2 07/21/2016 Roche Molecular Systems, Inc.
Cobas® EGFR Mutation Test, V2 And Cobas® CfDNA Sample Preparation Hungarian Translation Instruction... 2 05/26/2016 Roche Molecular Systems, Inc.
Hc2 System Software Suite 4.0 Version 3.0, Available As Component In Qiagen User Guide, Catalog #505... 2 06/26/2015 QIAGEN Gaithersburg, Inc.
Illumina MiSeqDx Universal Kit 1.0, PN 15039608 The Illumina MiSeqDx Universal Kit 1.0 Is A Set O... 2 12/23/2014 Illumina Inc
The BD MAX MRSA Assay, Catalogue #442953. An Automated Qualitative In Vitro Diagnostic Test For T... 2 09/10/2014 Becton Dickinson & Co.
Illumina Worklist Manager (IWM) (Software V1.0.15), A Component Of Illumina MiSeqDx Platform. Pro... 2 09/09/2014 Illumina Inc
Verigene Gram-Negative Blood Culture Nucleic Acid Test (BC-GN), Performed Using The Sample-To-Result... 2 08/08/2014 Nanosphere, Inc.
KIT Cobas 4800 HPV AMP/DET 240T / 960T US-IVD, CE-IVD Roche Molecular System, Inc. 1080 US Highway ... 2 09/10/2013 Roche Molecular Systems, Inc.
BD Affirm VPIII Microbial Identification Tests, Packaged In Kits, 120 Test\Kit, Catalog # 446257 And... 2 07/26/2013 Becton Dickinson & Co.
QIAGEN Artus CMV RG PCR ASR (96) (Catalog Number 4503225) Product Usage: Test Is Intended For U... 2 05/14/2013 QIAGEN Gaithersburg, Inc.
BD ProbeTec Neisseria Gonorrhoeae (GC) Qx Amplified DNA Assay Reagent Pack, REF 441124, Contains 12 ... 2 05/03/2013 Becton Dickinson & Co.
BD MAX PCR Cartridges Catalog #437519, Box Of 24 Cartridges Labeled In Part:***BD MAX PCR Cartridges... 2 03/22/2013 Becton Dickinson & Co.
BD GeneOhm MRSA ACP Assay, Catalog #441639, Box 200 Tests Labeled In Part***GeneOhm Sciences Canada,... 2 09/06/2012 Becton Dickinson & Co.
BD GeneOhm MRSA ACP Assay Catalog #441637, Box 48 Tests Labeled In Part***GeneOhm Sciences Canada, I... 2 09/06/2012 Becton Dickinson & Co.
BD GeneOhm Cdiff Assay , Catalog #441400 200, Box Tests 1-3 Labeled In Part***BD Diagnostics, 2555 B... 2 09/06/2012 Becton Dickinson & Co.
BD GeneOhm Cdiff Assay , Catalog # 441401, 200 Box Tests 2-3 Labeled In Part***GeneOhm Sciences C... 2 09/06/2012 Becton Dickinson & Co.
BD MAX GBS Catalog Number 441772 For In Vitro Diagnostic Use, For Use With The BD MAX System.The Sam... 2 09/16/2011 Becton Dickinson & Co.
BD MAX RNA Extraction Kit RNA-3, Catalog Number 437506. Kit Contains 24/2D Barcode Sample Preparatio... 2 09/16/2011 Becton Dickinson & Co.
BD MAX DNA Extraction Kit DNA-3 (Swabs In Transport Medium/UTM), Catalog Number 437503. Kit Contain... 2 09/16/2011 Becton Dickinson & Co.
BD MAX DNA Extraction Kit DNA-2 (Whole Blood) Catalog Number 437502. Kit Contains 24/2D Barcode Sam... 2 09/16/2011 Becton Dickinson & Co.
BD MAX DNA Extraction Kit DNA-1 (Urine/Plasma) Catalog Number 437501. Kit Contains 24/2D Barcode Sam... 2 09/16/2011 Becton Dickinson & Co.
XTAG CYP2D6 Kit V3 IVD For Use With Luminex 100/200 Instrument Luminex Molecular Diagnostics, Inc. 4... 2 08/31/2011 Luminex Corporation
MagNA Pure LC 2.0 (Software Version 1.1.23 And 1.1.24) Roche Diagnostics Operations, Inc. An Aut... 2 08/29/2011 Roche Diagnostics Operations, Inc.
MagNA Pure LC 1.0 (Software Version 3.0.11). Roche Diagnostics Operations, Inc. An Automated Ins... 2 08/29/2011 Roche Diagnostics Operations, Inc.
BD ProbeTec" CT/GC/AC Reagent Pack, Catalog #440450, Labeled In Part ***Becton Dickinson And Company... 2 06/24/2011 Becton Dickinson & Co.
Factor II (Prothrombin) G20210A Kit Light Cycler Instrument; Manufactured In Germany For Roche Molec... 2 06/14/2011 Roche Molecular Systems, Inc.
Factor V Leiden Kit Light Cycler Instrument; Manufactured In Germany For Roche Molecular Systems, In... 2 06/14/2011 Roche Molecular Systems, Inc.
Forteo [Teriparatide (RDNA Origin) Injection] Black Starter Kits Containing Triad Alcohol Pads. Th... 2 05/12/2011 Eli Lilly And Company
Bio Rad Brand Autoimmune EIA Anti-DsDNA Test Kit, 96 Tests, Catalog No. 96DS, Distributed By And Ma... 2 03/14/2011 Bio-Rad Laboratories Inc
Bio Rad Brand Autoimmune EIA Anti-DsDNA Test Kit, 576 Tests, Catalog No. 576DS, Distributed By And ... 2 03/14/2011 Bio-Rad Laboratories Inc
MagNA Pure LC 2.0 Instrument, Catalog Number 05197686001, Roche Diagnostics, Indianapolis, IN M... 2 03/14/2011 Roche Diagnostics Operations, Inc.
Factor II (Prothrombin) G20210A Kit, Catalog Number 03610195001, Roche Diagnostics, Indianapolis,... 2 03/14/2011 Roche Diagnostics Operations, Inc.
Factor V Leiden Kit Catalog Number 03610179001, Roche Diagnostics, Indianapolis, IN The Factor ... 2 03/14/2011 Roche Diagnostics Operations, Inc.
Quest EIA ANA Screen Bulk Kit, Model Number 96AN-BPU-QUEST. Manufactured By Bio-Rad Laboratories, I... 2 12/13/2010 Bio-Rad Laboratories Inc
DsDNA IgG ELISA 96 Well Kit, Catalog Number: DD037G The Products In Question Are All Enzyme Linke... 2 03/05/2010 Calbiotech Inc
BD Diagnostics, BD GeneOhm MRSA Assay, REF: 441242 (200 Reaction Kit) And 441244 (48 Reaction Kit). ... 2 09/10/2009 BD Diagnostics (GeneOhm Sciences, Inc)
RNAgents® Total RNA Isolation System (Cat. # Z5110). Kit Box Label/Box Drawer Label. For Laborato... 2 12/15/2008 Promega Corpopration
BD GeneOhm MRSA 48 Ct, Catalog #441244. IDI-MRSA Assay Is A Qualitative In Vitro Diagnostic Test Fo... 2 10/31/2008 BD Diagnostics (GeneOhm Sciences, Inc)
BD GeneOhm MRSA 200 Ct, Catalog #441242. IDI-MRSA Assay Is A Qualitative In Vitro Diagnostic Test ... 2 10/31/2008 BD Diagnostics (GeneOhm Sciences, Inc)
Roche MagNA Pure LC DNA Isolation Kit - Large Volume; Catalog Number 03310515001. 2 04/19/2006 Roche Diagnostics Corp.
Digene Hybrid Capture System CMV DNA Test (Version 2.0), Packaged In A Cardboard Box Containing Test... 2 07/20/2004 Digene Corp
Coulter DNA Prep Reagents Kit Part 6607055 2 11/19/2003 Beckman Coulter Inc
Digene''S Hybrid Capture 2 HPV DNA Test, Catalog # 5196-1230, Labeled For Export Only. 2 03/19/2003 Digene Corp
Digene''S Hybrid Capture 2 HPV DNA Test, Catalog # 5101-1096 2 03/19/2003 Digene Corp
Hybrid Capture 2 High-Risk HPV DNA Test, Catalog # 5101-1296 2 03/19/2003 Digene Corp
MagSIL (NucliSENS EasyMAG Magnetic Silica), Product Usage: The NucliSENS® EasyMAG® System Is An... 1 01/13/2017 BioMerieux SA
NucliSENS EasyMAG Magnetic Silica The NucliSENS EasyMAG Accessory Products Are Reagents And Dispo... 1 08/09/2016 BioMerieux SA
Cepheid Xpert MRSA/SA Blood Culture Test For Use With The GeneXpert Dx System, Manufactured By Cephe...
--------------------
https://en.wikipedia.org/wiki/Made_in_China_2025
NEWS IS IN THE MEDICAL INDUSTRY THAT THIS MEANS MEDICAL INSTRUMENTS (HIGH END ONES) WILL BE MADE IN CHINA BY 2025, THAT THE COMMUNIST GOVERNMENT HAS WRITTEN AN EDICT TO ORDER europe and usa et al to stop producing and buy these from china
--------------------
the fact is the medical community has "gotten greedy"
they give you an "FDA obama approved"\ rapid test, prescribe AWFUL MEDICINE (which has allot of dangers), and not care if the test was not 100% certain
they would never pull out the microscope and identify if the dna rna tester was right
(in a lab using such a device. in a normal lab with proper equipment that has microscopes: they WOULD properly identify before prescribing)
yet doctors often prescribe medication without CONCLUSIVE LAB RESULTS: that's an unfortunate fact and China knows it
infact: for "depression drugs" (which cause depression and suicide btw - contrary to the cause of prescription) - TENS OF MILLIONS OF AMERICANS HAVE BEEN PRESCRIBED DRUGS with absolutely zero lab work and no G.I. work done
(that is unrelated to dna rna tests - however - it figures in the big picture - it does very much so. so does the recall data)
BELIEVE ME, IF IT'S OBAMA CARE APPROVED, IF IT COSTS MONEY AND GOVERNMENT PAID TO CREATE A WEBSITE: IT'S A FRAUD OF SOME KIND
Cepheid
904 Caribbean Drive Sunnyvale, CA 94089 USA Phone: +1 408 541 4191 Fax: +1 408 541 4192
AH, so California is ALREADY GETTING GOVERNMENT FUNDS for private businesses. and lying about results no doubt. i guess they are also getting money for sales even though no one has asked for the product: i'm quite sure of it

LET ME TELL YOU ABOUT MOLECULAR TESTS - THEY DON'T COME IN A BOTTLE
YOU BUY A SUB-MILLION $ OR MULTI-MILLION $ LAB EQUIPMENT. You buy a data set (which comes with updates). the results (which use one of many standard solutions) have a data pattern - these patterns are compared against a known set (a prisoner up for murder dna, rna etc).
however - a dna rna tester REQUIRES your sample include the dna rna you are looking for (ie, not dna rna of SOMEONE ELSE)
the tests are not conclusive
WHAT THAT MEANS IS IF YOU ARE RAPID IDENTIFIED YOUR QUARANTEENED UNTIL YOU WAIT FOR ANOTHER TEST
it has nothing to do with buying a small bottle per say
IT IS A NJ BASED BUSINESS RUNNING IN CALIFORNIA? ALREADY SOME QUESTIONABLE LEADS.
Cost[edit]
Some concerns have been raised about the Xpert MTB/RIF, including minor operational issues and cost. The concessional price for a GeneXpert system is currently USD 32,000 for a four module instrument. As of 6 August 2012, the cost of a test cartridge in countries eligible for concessional pricing is USD 9.98.[11] As of 30 June 2013, 1,402 GeneXpert systems (comprising 7553 modules) and >3 million Xpert MTB/RIF cartridges had been procured in 88 of the 145 countries under concessional pricing
------------------------
most sunnyvale california companies have become MADE IN CHINA
they are called "fabless" and it means: they are talking heads, they are importers, they promote the product as american as long as they can get away with doing so
-------------------------
RECENT MEDICAL EQUIPMENT RECALLS
Product: dna
Results per Page
New Searchexport reports to excelExport To Excel | HelpHelp
Product DescriptionSort by Product Name [A-Z]
Sort by Product Name [Z-A] Recall
ClassSort by Recall Class [0-9]
Sort by Recall Class [9-0] FDA Recall
Posting DateSort by Date Classified [0-9]
Sort by Date Classified [9-0] Recalling FirmSort by Recalling Firm [A-Z]
Sort by Recalling Firm [Z-A]
DIASTAT(R) ANA (Anti-Nuclear Antibody) EURO DIAGNOSTICA, The DIASTAT(R) Anti-Nuclear Antibody (ANA) ... 3 10/11/2018 Euro Diagnostica AB
BD MAX DNA MMK Lab Use, Catalog No. 442828 3 04/28/2018 Becton Dickinson & Co.
BD MAX DNA MMK (SPC) For Laboratory Use, Catalog No. 442829 3 04/28/2018 Becton Dickinson & Co.
EnLite Neonatal TREC Kit;An In Vitro Diagnostic Device Intended For The Semi-Quantitative Determinat... 3 12/22/2016 PerkinElmer Health Sciences, Inc.
Thermo Scientific P21 WF1 Ab-3 (DCS-60.2) 1 Ml (0.4mg/Ml): Product Code: MS-230-P And MS-230P0; ... 3 11/20/2015 Lab Vision Corporation
Xpert CT/NG Urine Specimen Collection Kit Part Number GXCT/NGURINE-50; Microbiology: Xpert CT/NG... 3 08/20/2015 Cepheid
Xpert CT /NG Vaginal/Endocervical/ Specimen Collection Kit: Part Number CT/NGSWAB-50; Microbiolo... 3 08/20/2015 Cepheid
Cobas 4800 KRAS AMP/DET 24T CE-IVD Mutation Test; CE-IVD 5852170190. Intended For The Identificat... 3 06/26/2015 Roche Molecular Systems, Inc.
Cobas® EGFR Mutation Test Epidermal Growth Factor Receptor (EGFR) Gene DNA Assay 3 03/25/2015 Roche Molecular Systems, Inc.
The CEP 8 SpectrumGreen (SG) ASR Probe Kit, 20ul (List 06J37-018) Is An Analyte Specific Reagent (AS... 3 04/23/2014 Abbott Molecular
Cobas 4800 BRAF V600 Mutation Test For In Vitro Diagnostic Use Roche Molecular Systems, Inc., Pr... 3 11/02/2012 Roche Molecular Systems, Inc.
Cobas KRAS Mutation Test For In Vitro Diagnostic Use Product Usage: Usage: The Cobas KRAS Mutat... 3 08/08/2012 Roche Molecular Systems, Inc.
Abbott RealTime CT/NG Assay, List 8L07, In Vitro Diagnostic; Abbott Molecular Inc., Des Plaines, IL ... 3 02/23/2011 Abbott Molecular
Abbott RealTime HBV Assay, List 2N40, In Vitro Diagnostic; Abbott Molecular Inc., Des Plaines, IL 60... 3 02/23/2011 Abbott Molecular
Vysis LSI ATM/P53: D13S319 /CEP 12/13q34 DNA Probe Set; Fluorescence In Situ Hybridization (FISH) An... 3 03/13/2007 Abbott Molecular
Vysis LSI P16 (9p21)/CEP 9 (9p11-Q11) Dual Color Probe Set; A Locus Specific Identifier DNA Probe Co... 3 01/24/2006 Abbott Molecular
Anti-DsDNA [125I] Radiobinding Assay Kit. For In-Vitro Diagnostic Use Catalog Number: NEA 103 3 08/20/2003 Perkinelmer Life Sciences, Inc.
Kit BD Max ExK DNA 1 EU LUO; Catalog # 442818 2 08/22/2019 Becton Dickinson & Co.
Kit BD Max ExK DNA 1 USA; Catalog # 442817 2 08/22/2019 Becton Dickinson & Co.
Kit BD Max ExK DNA 2 USA; Catalog # 442819 2 08/22/2019 Becton Dickinson & Co.
Kit BD Max ExK DNA 2 EU LUO; Catalog # 442820 2 08/22/2019 Becton Dickinson & Co.
RNeasy DSP FFPE Kit (48), REF 73604 - Product Usage: The RNeasy DSP FFPE Kit Is A System Intended Fo... 2 06/28/2019 Qiagen Sciences, Inc.
Oncomine Dx Target Test, (Model No. A32451), Oncomine Dx Target RNA/DNA Panel (Model No. A32441). ... 2 04/20/2018 Life Technologies Corporation
Oncomine Dx Target Test User Guides And Assay Definition File, Model: A32461; UDI: (01)1019030200607... 2 03/30/2018 Life Technologies Corporation
DIASTAT Anti-Nuclear Antibody (ANA) / DIASTAT ANA ELISA. Catalog Number: FANA200. Product Usage: ... 2 03/08/2018 Euro Diagnostica AB
Dial-A-Dose Insulin Delivery Device (Pen-Injector) Cartridge Holders Product Usage: The NovoPen ... 2 12/07/2017 Novo Nordisk Inc
EMAG System, Ref 418591 It Is An In Vitro Diagnostic Medical Device Intended For The Automated Is... 2 09/12/2017 BioMerieux, Inc.
Welch Allyn ProBP 2400 Digital Blood Pressure Device #2400, REF 901096, Rx ONLY, Made In China --- D... 2 04/11/2017 Welch Allyn Inc
Cobas 6800/8800 System Hepatitis Viral B DNA Detection Product Usage: The Cobas P512 Pre-Analyt... 2 10/25/2016 Roche Diagnostics Operations, Inc.
Cobas EGFR Mutation Test V2 Materials Number CE-IVD: EGFR V2: 07248563190 CfDNA: 07247737190 ... 2 07/21/2016 Roche Molecular Systems, Inc.
Cobas® EGFR Mutation Test, V2 And Cobas® CfDNA Sample Preparation Hungarian Translation Instruction... 2 05/26/2016 Roche Molecular Systems, Inc.
Hc2 System Software Suite 4.0 Version 3.0, Available As Component In Qiagen User Guide, Catalog #505... 2 06/26/2015 QIAGEN Gaithersburg, Inc.
Illumina MiSeqDx Universal Kit 1.0, PN 15039608 The Illumina MiSeqDx Universal Kit 1.0 Is A Set O... 2 12/23/2014 Illumina Inc
The BD MAX MRSA Assay, Catalogue #442953. An Automated Qualitative In Vitro Diagnostic Test For T... 2 09/10/2014 Becton Dickinson & Co.
Illumina Worklist Manager (IWM) (Software V1.0.15), A Component Of Illumina MiSeqDx Platform. Pro... 2 09/09/2014 Illumina Inc
Verigene Gram-Negative Blood Culture Nucleic Acid Test (BC-GN), Performed Using The Sample-To-Result... 2 08/08/2014 Nanosphere, Inc.
KIT Cobas 4800 HPV AMP/DET 240T / 960T US-IVD, CE-IVD Roche Molecular System, Inc. 1080 US Highway ... 2 09/10/2013 Roche Molecular Systems, Inc.
BD Affirm VPIII Microbial Identification Tests, Packaged In Kits, 120 Test\Kit, Catalog # 446257 And... 2 07/26/2013 Becton Dickinson & Co.
QIAGEN Artus CMV RG PCR ASR (96) (Catalog Number 4503225) Product Usage: Test Is Intended For U... 2 05/14/2013 QIAGEN Gaithersburg, Inc.
BD ProbeTec Neisseria Gonorrhoeae (GC) Qx Amplified DNA Assay Reagent Pack, REF 441124, Contains 12 ... 2 05/03/2013 Becton Dickinson & Co.
BD MAX PCR Cartridges Catalog #437519, Box Of 24 Cartridges Labeled In Part:***BD MAX PCR Cartridges... 2 03/22/2013 Becton Dickinson & Co.
BD GeneOhm MRSA ACP Assay, Catalog #441639, Box 200 Tests Labeled In Part***GeneOhm Sciences Canada,... 2 09/06/2012 Becton Dickinson & Co.
BD GeneOhm MRSA ACP Assay Catalog #441637, Box 48 Tests Labeled In Part***GeneOhm Sciences Canada, I... 2 09/06/2012 Becton Dickinson & Co.
BD GeneOhm Cdiff Assay , Catalog #441400 200, Box Tests 1-3 Labeled In Part***BD Diagnostics, 2555 B... 2 09/06/2012 Becton Dickinson & Co.
BD GeneOhm Cdiff Assay , Catalog # 441401, 200 Box Tests 2-3 Labeled In Part***GeneOhm Sciences C... 2 09/06/2012 Becton Dickinson & Co.
BD MAX GBS Catalog Number 441772 For In Vitro Diagnostic Use, For Use With The BD MAX System.The Sam... 2 09/16/2011 Becton Dickinson & Co.
BD MAX RNA Extraction Kit RNA-3, Catalog Number 437506. Kit Contains 24/2D Barcode Sample Preparatio... 2 09/16/2011 Becton Dickinson & Co.
BD MAX DNA Extraction Kit DNA-3 (Swabs In Transport Medium/UTM), Catalog Number 437503. Kit Contain... 2 09/16/2011 Becton Dickinson & Co.
BD MAX DNA Extraction Kit DNA-2 (Whole Blood) Catalog Number 437502. Kit Contains 24/2D Barcode Sam... 2 09/16/2011 Becton Dickinson & Co.
BD MAX DNA Extraction Kit DNA-1 (Urine/Plasma) Catalog Number 437501. Kit Contains 24/2D Barcode Sam... 2 09/16/2011 Becton Dickinson & Co.
XTAG CYP2D6 Kit V3 IVD For Use With Luminex 100/200 Instrument Luminex Molecular Diagnostics, Inc. 4... 2 08/31/2011 Luminex Corporation
MagNA Pure LC 2.0 (Software Version 1.1.23 And 1.1.24) Roche Diagnostics Operations, Inc. An Aut... 2 08/29/2011 Roche Diagnostics Operations, Inc.
MagNA Pure LC 1.0 (Software Version 3.0.11). Roche Diagnostics Operations, Inc. An Automated Ins... 2 08/29/2011 Roche Diagnostics Operations, Inc.
BD ProbeTec" CT/GC/AC Reagent Pack, Catalog #440450, Labeled In Part ***Becton Dickinson And Company... 2 06/24/2011 Becton Dickinson & Co.
Factor II (Prothrombin) G20210A Kit Light Cycler Instrument; Manufactured In Germany For Roche Molec... 2 06/14/2011 Roche Molecular Systems, Inc.
Factor V Leiden Kit Light Cycler Instrument; Manufactured In Germany For Roche Molecular Systems, In... 2 06/14/2011 Roche Molecular Systems, Inc.
Forteo [Teriparatide (RDNA Origin) Injection] Black Starter Kits Containing Triad Alcohol Pads. Th... 2 05/12/2011 Eli Lilly And Company
Bio Rad Brand Autoimmune EIA Anti-DsDNA Test Kit, 96 Tests, Catalog No. 96DS, Distributed By And Ma... 2 03/14/2011 Bio-Rad Laboratories Inc
Bio Rad Brand Autoimmune EIA Anti-DsDNA Test Kit, 576 Tests, Catalog No. 576DS, Distributed By And ... 2 03/14/2011 Bio-Rad Laboratories Inc
MagNA Pure LC 2.0 Instrument, Catalog Number 05197686001, Roche Diagnostics, Indianapolis, IN M... 2 03/14/2011 Roche Diagnostics Operations, Inc.
Factor II (Prothrombin) G20210A Kit, Catalog Number 03610195001, Roche Diagnostics, Indianapolis,... 2 03/14/2011 Roche Diagnostics Operations, Inc.
Factor V Leiden Kit Catalog Number 03610179001, Roche Diagnostics, Indianapolis, IN The Factor ... 2 03/14/2011 Roche Diagnostics Operations, Inc.
Quest EIA ANA Screen Bulk Kit, Model Number 96AN-BPU-QUEST. Manufactured By Bio-Rad Laboratories, I... 2 12/13/2010 Bio-Rad Laboratories Inc
DsDNA IgG ELISA 96 Well Kit, Catalog Number: DD037G The Products In Question Are All Enzyme Linke... 2 03/05/2010 Calbiotech Inc
BD Diagnostics, BD GeneOhm MRSA Assay, REF: 441242 (200 Reaction Kit) And 441244 (48 Reaction Kit). ... 2 09/10/2009 BD Diagnostics (GeneOhm Sciences, Inc)
RNAgents® Total RNA Isolation System (Cat. # Z5110). Kit Box Label/Box Drawer Label. For Laborato... 2 12/15/2008 Promega Corpopration
BD GeneOhm MRSA 48 Ct, Catalog #441244. IDI-MRSA Assay Is A Qualitative In Vitro Diagnostic Test Fo... 2 10/31/2008 BD Diagnostics (GeneOhm Sciences, Inc)
BD GeneOhm MRSA 200 Ct, Catalog #441242. IDI-MRSA Assay Is A Qualitative In Vitro Diagnostic Test ... 2 10/31/2008 BD Diagnostics (GeneOhm Sciences, Inc)
Roche MagNA Pure LC DNA Isolation Kit - Large Volume; Catalog Number 03310515001. 2 04/19/2006 Roche Diagnostics Corp.
Digene Hybrid Capture System CMV DNA Test (Version 2.0), Packaged In A Cardboard Box Containing Test... 2 07/20/2004 Digene Corp
Coulter DNA Prep Reagents Kit Part 6607055 2 11/19/2003 Beckman Coulter Inc
Digene''S Hybrid Capture 2 HPV DNA Test, Catalog # 5196-1230, Labeled For Export Only. 2 03/19/2003 Digene Corp
Digene''S Hybrid Capture 2 HPV DNA Test, Catalog # 5101-1096 2 03/19/2003 Digene Corp
Hybrid Capture 2 High-Risk HPV DNA Test, Catalog # 5101-1296 2 03/19/2003 Digene Corp
MagSIL (NucliSENS EasyMAG Magnetic Silica), Product Usage: The NucliSENS® EasyMAG® System Is An... 1 01/13/2017 BioMerieux SA
NucliSENS EasyMAG Magnetic Silica The NucliSENS EasyMAG Accessory Products Are Reagents And Dispo... 1 08/09/2016 BioMerieux SA
Cepheid Xpert MRSA/SA Blood Culture Test For Use With The GeneXpert Dx System, Manufactured By Cephe...
--------------------
https://en.wikipedia.org/wiki/Made_in_China_2025
NEWS IS IN THE MEDICAL INDUSTRY THAT THIS MEANS MEDICAL INSTRUMENTS (HIGH END ONES) WILL BE MADE IN CHINA BY 2025, THAT THE COMMUNIST GOVERNMENT HAS WRITTEN AN EDICT TO ORDER europe and usa et al to stop producing and buy these from china
--------------------
the fact is the medical community has "gotten greedy"
they give you an "FDA obama approved"\ rapid test, prescribe AWFUL MEDICINE (which has allot of dangers), and not care if the test was not 100% certain
they would never pull out the microscope and identify if the dna rna tester was right
(in a lab using such a device. in a normal lab with proper equipment that has microscopes: they WOULD properly identify before prescribing)
yet doctors often prescribe medication without CONCLUSIVE LAB RESULTS: that's an unfortunate fact and China knows it
infact: for "depression drugs" (which cause depression and suicide btw - contrary to the cause of prescription) - TENS OF MILLIONS OF AMERICANS HAVE BEEN PRESCRIBED DRUGS with absolutely zero lab work and no G.I. work done
(that is unrelated to dna rna tests - however - it figures in the big picture - it does very much so. so does the recall data)
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
r4fgtbgrew
()
Date: March 22, 2020 05:28PM
there is no precise estimation of the number of commercially available RIDTs because of the growing number of test kits made in China and other southeastern Asian countries
Assay performance has been evaluated in 12 clinical studies, of which 10 were published in peer-reviewed journals (TABLE 2) and 2 were presented as posters in scientific meetings. For the Xpert Flu V3, sensitivity varied from 76.9 to 98.1%, but specificity was above 97% in all studies. For Flu A (Xpert Flu V1 and V2), sensitivity varied from 64.3 to 98.7%. The low value reported previously [26] was attributable to the inclusion of H9N2 and H5N1 that were either not detected (0/3) or only partially detected (1/3), respectively (TABLE 2). It is noteworthy that the Xpert Flu assay had not been developed for detection of these strains. Advances to include results based on these subtypes into the calculation of analytical sensitivity are therefore vulnerable. Most of the studies showed sensitivity above 413
Assay performance has been evaluated in 12 clinical studies, of which 10 were published in peer-reviewed journals (TABLE 2) and 2 were presented as posters in scientific meetings. For the Xpert Flu V3, sensitivity varied from 76.9 to 98.1%, but specificity was above 97% in all studies. For Flu A (Xpert Flu V1 and V2), sensitivity varied from 64.3 to 98.7%. The low value reported previously [26] was attributable to the inclusion of H9N2 and H5N1 that were either not detected (0/3) or only partially detected (1/3), respectively (TABLE 2). It is noteworthy that the Xpert Flu assay had not been developed for detection of these strains. Advances to include results based on these subtypes into the calculation of analytical sensitivity are therefore vulnerable. Most of the studies showed sensitivity above 413
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
rfgbgrere
()
Date: March 22, 2020 05:29PM
https://en.wikipedia.org/wiki/Made_in_China_2025
The Center for Strategic and International Studies describes it as an "initiative to comprehensively upgrade Chinese industry" directly inspired by the German Industry 4.0.
(USA petitions to prevent drug imports because, among other things, foreigners are using us patents without paying, are "un-controlled", AND COMPETE UNFAIRLY in the free market: it's a communist market globally)
The Center for Strategic and International Studies describes it as an "initiative to comprehensively upgrade Chinese industry" directly inspired by the German Industry 4.0.
(USA petitions to prevent drug imports because, among other things, foreigners are using us patents without paying, are "un-controlled", AND COMPETE UNFAIRLY in the free market: it's a communist market globally)
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
breitbart
()
Date: March 22, 2020 05:36PM
i'm not 100% certain (i'm, say, 76.9% certain) that GeneXpert is made in China
however i have experience in chasing these things down and have allot of reasons to suspect it IS MADE IN CHINA
AND THAT OBAMA OFFICIALS IN FDA ARE HELPING CHINA OVER-THROW THE US MEDICAL EQUIPMENT INDUSTRY - actually using federal money to create websites for them
(note: websites contracts since bill clinton are something like $2,000,000 year just for a crappy web site a teenager could make: the contract always is steered corruptly of course)
however i have experience in chasing these things down and have allot of reasons to suspect it IS MADE IN CHINA
AND THAT OBAMA OFFICIALS IN FDA ARE HELPING CHINA OVER-THROW THE US MEDICAL EQUIPMENT INDUSTRY - actually using federal money to create websites for them
(note: websites contracts since bill clinton are something like $2,000,000 year just for a crappy web site a teenager could make: the contract always is steered corruptly of course)
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
breitbart
()
Date: March 22, 2020 05:42PM
https://www.breitbart.com/border/2020/03/21/rapid-coronavirus-test-approved-by-fda-results-in-45-minutes/
order one now: it's federal money who cares how it's spent !!
order one now: it's federal money who cares how it's spent !!
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
09sdfj
()
Date: March 22, 2020 05:58PM
however - a dna rna tester REQUIRES your sample include the dna rna you are looking for (ie, not dna rna of SOMEONE ELSE)
i have no idea if the Xpert system is new and different but i severely doubt it
WHAT IT MEANS IS YOU HAVE TO HAVE IDENTIFIED A sample (isolated it, meaning you already know it's there and have collected it) to put it in the machina
To quantify gene expression, the (Cq) for an RNA or DNA from the gene of interest is subtracted from the (Cq) of RNA/DNA from a housekeeping gene in the same sample to normalize for variation in the amount and quality of RNA between different samples. This normalization procedure is commonly called the Ct-method[14] and permits comparison of expression of a gene of interest among different samples. However, for such comparison, expression of the normalizing reference gene needs to be very similar across all the samples. Choosing a reference gene fulfilling this criterion is therefore of high importance, and often challenging, because only very few genes show equal levels of expression across a range of different conditions or tissues
-----------------------------------
a typical dna rna comparator (for prison use) doesn't really identify genes: the sample is taken from known human dna and compared (the meaning of the data is not significant and infact the "color data" is mostly useless medically - only the comparison between one person and another is). if you took a prisoner sample containing dog blood and another sample also containing dog blood there would be a match: although lab persons could probably say it was dog dna - the point remains THE RIGHT STUFF NEEDS TO BE PUT IN THE TEST CHAMBERS. the color data IS NOT STANDARDIZED per say - different machines produce different and incompatible color data (there are some conversions but not always - you many need the same system or style of system to compare with)
---------------------------
there is another kind of system - rapid identification system - that "never took off". it visually identifies virus and microbes and pathogen using a microscope and automated visual comparison. in early 2000's these systems were deemed "too slow to be practical" (that a qualified lab person could do it faster)
-----------------------------
while i don't know a great amount about these systems - i know to look out for corruption IN EVERY CORNER OF EVERY MODERN SYSTEM (government) has had a hand in promoting
there is nothing sacred these days: these systems cost a ton of money even if they are not bought they are paid for by the taxpayer - even if they were made in china
i have no idea if the Xpert system is new and different but i severely doubt it
WHAT IT MEANS IS YOU HAVE TO HAVE IDENTIFIED A sample (isolated it, meaning you already know it's there and have collected it) to put it in the machina
if you already know the sample contains covid19, it is unlikely you need a bot to tell you it is.
why is because there thousands of little pathogens running around in your blood
the question is which one would you test? it's not going to be 45 min. if you need to test all of them, that's for sure
To quantify gene expression, the (Cq) for an RNA or DNA from the gene of interest is subtracted from the (Cq) of RNA/DNA from a housekeeping gene in the same sample to normalize for variation in the amount and quality of RNA between different samples. This normalization procedure is commonly called the Ct-method[14] and permits comparison of expression of a gene of interest among different samples. However, for such comparison, expression of the normalizing reference gene needs to be very similar across all the samples. Choosing a reference gene fulfilling this criterion is therefore of high importance, and often challenging, because only very few genes show equal levels of expression across a range of different conditions or tissues
-----------------------------------
a typical dna rna comparator (for prison use) doesn't really identify genes: the sample is taken from known human dna and compared (the meaning of the data is not significant and infact the "color data" is mostly useless medically - only the comparison between one person and another is). if you took a prisoner sample containing dog blood and another sample also containing dog blood there would be a match: although lab persons could probably say it was dog dna - the point remains THE RIGHT STUFF NEEDS TO BE PUT IN THE TEST CHAMBERS. the color data IS NOT STANDARDIZED per say - different machines produce different and incompatible color data (there are some conversions but not always - you many need the same system or style of system to compare with)
---------------------------
there is another kind of system - rapid identification system - that "never took off". it visually identifies virus and microbes and pathogen using a microscope and automated visual comparison. in early 2000's these systems were deemed "too slow to be practical" (that a qualified lab person could do it faster)
-----------------------------
while i don't know a great amount about these systems - i know to look out for corruption IN EVERY CORNER OF EVERY MODERN SYSTEM (government) has had a hand in promoting
there is nothing sacred these days: these systems cost a ton of money even if they are not bought they are paid for by the taxpayer - even if they were made in china
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
made in china dot com
()
Date: March 22, 2020 06:02PM
it's a "Real-time PCR system"
china has an endless number of competing PCR systems

one of the tens of real time pcr systems "out there"
china has an endless number of competing PCR systems

one of the tens of real time pcr systems "out there"
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
TRUMPOCRAP
()
Date: March 22, 2020 06:03PM
WHAT THE FUCK ARE YOU TRYING TO SAY!
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
4rfgrtbere
()
Date: March 22, 2020 06:05PM
The Gene Xpert is a machine that can detect *mycobacterium tuberculosis* in a sample of sputum. A person suspected of having TB needs to give a sputum sample, which the health care worker than places in a small tube.
THERE IS LITTLE CHANCE THE PATIENT HAS SPUTIN TO PUT IN A SAMPLE JAR IF THEY DON'T HAVE TB
see - there is your problem
THERE IS LITTLE CHANCE THE PATIENT HAS SPUTIN TO PUT IN A SAMPLE JAR IF THEY DON'T HAVE TB
see - there is your problem
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
4rfgrtbere
()
Date: March 22, 2020 06:07PM
mountains of manuals - two important points to find:
#1 is it a "SEMI" fraud of some kind ? it can't do what it says it will ?
#2 is it a fabless country using usa money to sell chinese stuff ?
#1 is it a "SEMI" fraud of some kind ? it can't do what it says it will ?
#2 is it a fabless country using usa money to sell chinese stuff ?
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
4rfgrtbere
()
Date: March 22, 2020 06:10PM
let's say - it's not the first time i've shopped around - and mountains of manuals seem to have lied to me before so i have some idea where to look
anyone my age should "know the routine" when shopping, if money matters
----------------------------------
Unsuccessful Xpert MTB/RIF results: the Nigerian experiencewww.ncbi.nlm.nih.gov pmc articles PMC5858060
by M Gidado - 2018 - Cited by 12 - Related articles
Mar 21, 2018 - The resolution of error results was dependent on the error codes. Problematic issues that persisted after troubleshooting were reported to the ..
anyone my age should "know the routine" when shopping, if money matters
----------------------------------
Unsuccessful Xpert MTB/RIF results: the Nigerian experiencewww.ncbi.nlm.nih.gov pmc articles PMC5858060
by M Gidado - 2018 - Cited by 12 - Related articles
Mar 21, 2018 - The resolution of error results was dependent on the error codes. Problematic issues that persisted after troubleshooting were reported to the ..
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
r4tgbgre
()
Date: March 22, 2020 06:12PM
Methods: We are reporting serious issues with repeatability among a subgroup of Xpert (Version 4) identified RR results from South Indian state ...
and the good news is in 2020: there is so much fake news you can't verify product complaints because those also are fake.
asians employ asians to create fake product reports against competitors and fake glowering reports (using other people's names) of their own products
------------------------
... try before you BUY
and the good news is in 2020: there is so much fake news you can't verify product complaints because those also are fake.
asians employ asians to create fake product reports against competitors and fake glowering reports (using other people's names) of their own products
------------------------
... try before you BUY
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
k
()
Date: March 22, 2020 06:28PM
i guess you have to guess or realize that the machine that "takes any sample and forays all dna and rna of all types and identifies them doesn't exist" before you start...
you'd know this if you'd looked into several types of lab equipment and what they do (ie, atomic versus covalent bonding and types of early dna machines and types of)
you could then guess that a machine claiming "that since yesterday it can identify covid19" rapidly from "a sample" (you'd think blood - but then there is the TRICK) in just 45 min "right at the time federal money is available"
... might contain some false advertising
and probably does
you'd know this if you'd looked into several types of lab equipment and what they do (ie, atomic versus covalent bonding and types of early dna machines and types of)
you could then guess that a machine claiming "that since yesterday it can identify covid19" rapidly from "a sample" (you'd think blood - but then there is the TRICK) in just 45 min "right at the time federal money is available"
... might contain some false advertising
and probably does
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
k
()
Date: March 22, 2020 06:37PM
(most machines that "identify complex substances" can only differentiate or identify many substances from one another: and completely fail if the wrong sample is put in. how do you get your sample: allot of work)
https://med.stanford.edu/news/all-news/2020/03/stanford-medicine-COVID-19-test-now-in-use.html (guess what, another PCR method requiring isolation before test)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2772359/
ABSTRACT
Summary: Electron microscopy, considered by some to be an old technique, is still on the forefront of both clinical viral diagnoses and viral ultrastructure and pathogenesis studies. In the diagnostic setting, it is particularly valuable in the surveillance of emerging diseases and potential bioterrorism viruses. In the research arena, modalities such as immunoelectron microscopy, cryo-electron microscopy, and electron tomography have demonstrated how viral structural components fit together, attach to cells, assimilate during replication, and associate with the cellular machinery during replication and egression. These studies provide information for treatment and vaccine strategies.
One of the main advantages of using EM for viral diagnosis is that it does not require organism-specific reagents for recognizing the pathogenic agent. Other tests involving molecular and serological methods require that a specific probe be available for virus identification. In the event of a disease caused by an unknown pathogen, it is hard to know which reagent to pick. On the other hand, EM allows an “open view” (a term coined by Hans Gelderblom) of whatever might be present, while molecular tests require knowledge about the potential agent(s) to determine the correct test(s). EM, though it may not be able to identify a virus beyond the family level, at least points the way for more specific identification by other methods such as biochemical assays for specific pathogens. Another fact to keep in mind is that reagents do not exist for all viruses; when they grow poorly or not at all in in vitro systems, obtaining enough material to produce commercial test kits is difficult. Finally, in cases of dual infections, molecular or antigen-based testing would likely miss the second agent.
Even today, in the age of molecular diagnostics, EM is a mainstay in detecting new and unusual outbreaks. For example, norovirus (Norwalk agent) was discovered by EM (46), and EM continues to serve to confirm infection in quality control of molecular techniques (87). EM was instrumental in elucidating the viral agent of the first outbreak of Ebola virus in Zaire in 1976 (8, 45, 71) and in identifying the Ebola Reston infection of a monkey colony in Reston, VA, in 1989 as being caused by a filovirus
https://med.stanford.edu/news/all-news/2020/03/stanford-medicine-COVID-19-test-now-in-use.html (guess what, another PCR method requiring isolation before test)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2772359/
ABSTRACT
Summary: Electron microscopy, considered by some to be an old technique, is still on the forefront of both clinical viral diagnoses and viral ultrastructure and pathogenesis studies. In the diagnostic setting, it is particularly valuable in the surveillance of emerging diseases and potential bioterrorism viruses. In the research arena, modalities such as immunoelectron microscopy, cryo-electron microscopy, and electron tomography have demonstrated how viral structural components fit together, attach to cells, assimilate during replication, and associate with the cellular machinery during replication and egression. These studies provide information for treatment and vaccine strategies.
One of the main advantages of using EM for viral diagnosis is that it does not require organism-specific reagents for recognizing the pathogenic agent. Other tests involving molecular and serological methods require that a specific probe be available for virus identification. In the event of a disease caused by an unknown pathogen, it is hard to know which reagent to pick. On the other hand, EM allows an “open view” (a term coined by Hans Gelderblom) of whatever might be present, while molecular tests require knowledge about the potential agent(s) to determine the correct test(s). EM, though it may not be able to identify a virus beyond the family level, at least points the way for more specific identification by other methods such as biochemical assays for specific pathogens. Another fact to keep in mind is that reagents do not exist for all viruses; when they grow poorly or not at all in in vitro systems, obtaining enough material to produce commercial test kits is difficult. Finally, in cases of dual infections, molecular or antigen-based testing would likely miss the second agent.
Even today, in the age of molecular diagnostics, EM is a mainstay in detecting new and unusual outbreaks. For example, norovirus (Norwalk agent) was discovered by EM (46), and EM continues to serve to confirm infection in quality control of molecular techniques (87). EM was instrumental in elucidating the viral agent of the first outbreak of Ebola virus in Zaire in 1976 (8, 45, 71) and in identifying the Ebola Reston infection of a monkey colony in Reston, VA, in 1989 as being caused by a filovirus
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
k
()
Date: March 22, 2020 06:47PM
Sara E. Miller
Infectious Disease Pathology Branch, Centers for Disease Control and Prevention, Atlanta, Georgia,1 Department of Pathology, Duke University Medical Center, Durham, North Carolina2
Cynthia S. Goldsmith
Infectious Disease Pathology Branch, Centers for Disease Control and Prevention, Atlanta, Georgia,1 Department of Pathology, Duke University Medical Center, Durham, North Carolina2
Numerous atlases (2, 17, 18, 22, 41, 56, 57, 69, 70) and websites with excellent micrographs for virus identification by negative staining and thin sectioning are available. There are also online pictures of viruses (http://www.virology.net/Big_Virology/BVHomePage.html, http://www.virology.net/garryfavweb12.html).
Caveats
In fluid samples, the concentration of viruses has to be high for detection. Liquids should routinely be ultracentrifuged, after first clarifying them at low speed, to pellet and concentrate viruses. Other concentration methods, including ultrafiltration, agar diffusion, pseudoreplica technique, and immunoaggregation have been reviewed
(the operator must be skilled to not mis-identify - to know the likenesses and pitfalls causing mis-identification)
INFACT - THE CDC HAS RELEASED EM PHOTOS (for use with this)
----------------------------------
meanwhile
https://med.stanford.edu/news/all-news/2020/03/stanford-medicine-COVID-19-test-now-in-use.html
i tried the weblinks - the lead to covid hype pages but not the procedures suggested ... such as why 3 separate tests are needed and what is that 3rd test
Infectious Disease Pathology Branch, Centers for Disease Control and Prevention, Atlanta, Georgia,1 Department of Pathology, Duke University Medical Center, Durham, North Carolina2
Cynthia S. Goldsmith
Infectious Disease Pathology Branch, Centers for Disease Control and Prevention, Atlanta, Georgia,1 Department of Pathology, Duke University Medical Center, Durham, North Carolina2
Numerous atlases (2, 17, 18, 22, 41, 56, 57, 69, 70) and websites with excellent micrographs for virus identification by negative staining and thin sectioning are available. There are also online pictures of viruses (http://www.virology.net/Big_Virology/BVHomePage.html, http://www.virology.net/garryfavweb12.html).
Caveats
In fluid samples, the concentration of viruses has to be high for detection. Liquids should routinely be ultracentrifuged, after first clarifying them at low speed, to pellet and concentrate viruses. Other concentration methods, including ultrafiltration, agar diffusion, pseudoreplica technique, and immunoaggregation have been reviewed
(the operator must be skilled to not mis-identify - to know the likenesses and pitfalls causing mis-identification)
INFACT - THE CDC HAS RELEASED EM PHOTOS (for use with this)
----------------------------------
meanwhile
https://med.stanford.edu/news/all-news/2020/03/stanford-medicine-COVID-19-test-now-in-use.html
i tried the weblinks - the lead to covid hype pages but not the procedures suggested ... such as why 3 separate tests are needed and what is that 3rd test
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
korrection
()
Date: March 22, 2020 06:51PM
hype pages meaning "college news by college students", and the links lead to "password only healthcare local website"
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
fgtbrew
()
Date: March 22, 2020 06:53PM
Figure1 Diagnosisofvirusinfectionsbyexaminationofultrathinsectionsofhumantissuesorcells
(A) Parapoxvirus (Orf virus) infection on a human skin biopsy specimen. Multiple oval viral particles (arrow), comprising a dense core surrounded by an envelope (inset, high magnification), are observed in an infected cell. The Orf virus is a parapoxivirus that causes a common skin disease of sheep and goats,
AH OK - THEN EM IS GOAT APPROVED THEN
(A) Parapoxvirus (Orf virus) infection on a human skin biopsy specimen. Multiple oval viral particles (arrow), comprising a dense core surrounded by an envelope (inset, high magnification), are observed in an infected cell. The Orf virus is a parapoxivirus that causes a common skin disease of sheep and goats,
AH OK - THEN EM IS GOAT APPROVED THEN
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
tan, stain, and brimmer
()
Date: March 22, 2020 06:54PM
an anthology of EM
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
dear reader
()
Date: March 22, 2020 06:58PM

the reader should realize that "ALLOT OF PUBLISHED SCIENCE REVOLVES AROUND DEVELOPING THE BETTER MICROSCOPE" (and who will steal the data and produce it and sell it to governments)
that is no joke. any college lab worth mentioning has not one but many "better microscope" projects ongoing
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
k
()
Date: March 22, 2020 07:01PM
if you have a dead patient, your a coroner with a midline microscope, you see bacteria in the lungs shouldn't be there and know of symptoms: you say "it's covid19" and send a sample to the feds
if you have a healthy patient you likely aren't allowed to take lung samples "just to see", so you have blood samples - and that's not going to be easy - from what i hear
job done!
if you have a healthy patient you likely aren't allowed to take lung samples "just to see", so you have blood samples - and that's not going to be easy - from what i hear
job done!
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
rgf0e9podf
()
Date: March 22, 2020 07:07PM
that is no joke. any college lab worth mentioning has not one but many "better microscope" projects ongoing ...
this is where chinese walking out of usa schools with "lab data" gets in the news
germany is a good creator of science - but often takes it as well ...
germany and japan are currently ON THE TOP of micoscopy SALES
this is where chinese walking out of usa schools with "lab data" gets in the news
germany is a good creator of science - but often takes it as well ...
germany and japan are currently ON THE TOP of micoscopy SALES
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
rgf0e9podf
()
Date: March 22, 2020 07:07PM
to say china is on their tales would be an under-statement
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
k
()
Date: March 22, 2020 07:09PM
little red riding hood
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
k
()
Date: March 22, 2020 07:19PM

there are systems designed to look at EM photos for rapid identification - but they are slow, have a thin database of "known likenesses" to identify, and it takes "some intelligence" to identify certain things from certain other things in photos
they are "still in the works" - meaning a lab person is usually better off not having EM rapid identification software
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
rfgrew
()
Date: March 22, 2020 07:24PM
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
98sdfhf
()
Date: March 22, 2020 07:45PM
Polymerase chain reaction (PCR) is a method widely used in molecular biology to rapidly make millions to billions of copies of a specific DNA sample allowing scientists to take a very small sample of DNA and amplify it to a large enough amount to study in detail. PCR was invented in 1983 by Kary Mullis. It is fundamental to much of genetic testing including analysis of ancient samples of DNA and identification of infectious agents. Using PCR, copies of very small amounts of DNA sequences are exponentially amplified in a series or cycles of temperature changes. PCR is now a common and often indispensable technique used in medical laboratory and clinical laboratory research for a broad variety of applications including biomedical research and criminal forensics
AH - SO IF PCR IS YOUR FIRST STEP - YOU ARE ADMITTING TO "MANUFACURING THE CORONAVIRUS COVID-19 INTENTIONALLY" ??
.
AH - SO IF PCR IS YOUR FIRST STEP - YOU ARE ADMITTING TO "MANUFACURING THE CORONAVIRUS COVID-19 INTENTIONALLY" ??
.
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
CORRECTION
()
Date: March 22, 2020 07:50PM
THAT'S JUST A VERY POOR WIKIPEDIA EXPLINATION - cancel the 98sdfhf post immediately above
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
PCR
()
Date: March 22, 2020 07:52PM
(pcr begins by "uncoiling the helix" - a method that was used in the earliest dna tests but was not given that name. the next step in the old method was to use electrical charge to pull the strand across a gel and note the results - yielding "color bars", a simple process that has since been refined in many ways)
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
rfgbgrerw
()
Date: March 22, 2020 07:54PM
Other than denaturation by heat, nucleic acids can undergo the denaturation process through various chemical agents such as XXX
in other words, these pcr machines having title "MJ Research PTC-200 DNA Engine 96-well PCR Unit, Tested Working !!"
ARE JUST HOT PADS, you can say
in other words, these pcr machines having title "MJ Research PTC-200 DNA Engine 96-well PCR Unit, Tested Working !!"
ARE JUST HOT PADS, you can say
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
fun facts
()
Date: March 22, 2020 08:07PM
the .5-5 micron above means 1 micro meter = 10,000 angstrom

(remembering microscopes and dna color bar machines are sciences "better mouse trap")
this "older" image is of 5 atoms - so it's a molecule of course. the thing to note is it's resolution isn't "5 A" - it's more than that because you can make out details within the 5A can't you? sure you can see quite a picture
talk about resolution !

(remembering microscopes and dna color bar machines are sciences "better mouse trap")
this "older" image is of 5 atoms - so it's a molecule of course. the thing to note is it's resolution isn't "5 A" - it's more than that because you can make out details within the 5A can't you? sure you can see quite a picture
talk about resolution !
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
Show Off !!
()
Date: March 22, 2020 08:08PM

Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
5 A
()
Date: March 22, 2020 08:29PM
the atom is roughly .5 A to 2 or a few A for the larger ones - though wikipedia has "BANNED ALL USE OF ANGRSTROM" - if you hadn't heard. edited all out by moderators. try to post it: you will be deleted
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
Show Off !!
()
Date: March 22, 2020 08:32PM
20 nm is 200A, so the L's are about 200 atoms wide - which is wider than a circuit on a modern Intel chip btw
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
09sdfjf
()
Date: March 22, 2020 08:34PM
Show Off !! Wrote:
-------------------------------------------------------
> 20 nm is 200A, so the L's are about 200 atoms wide
> - which is wider than a circuit on a modern Intel
> chip btw
doesn't matter - the chinese got the publication - you'll be out of business soon !!
-------------------------------------------------------
> 20 nm is 200A, so the L's are about 200 atoms wide
> - which is wider than a circuit on a modern Intel
> chip btw
doesn't matter - the chinese got the publication - you'll be out of business soon !!
Re: more phonies than virus cases: We’re authorizing today will be able to provide Americans with results within 45 min approved by FDA XpertXpress Sars-CoV-2 rapid molecular diagnostic test
Posted by:
k
()
Date: April 03, 2020 07:21AM
TOTAL RECALL